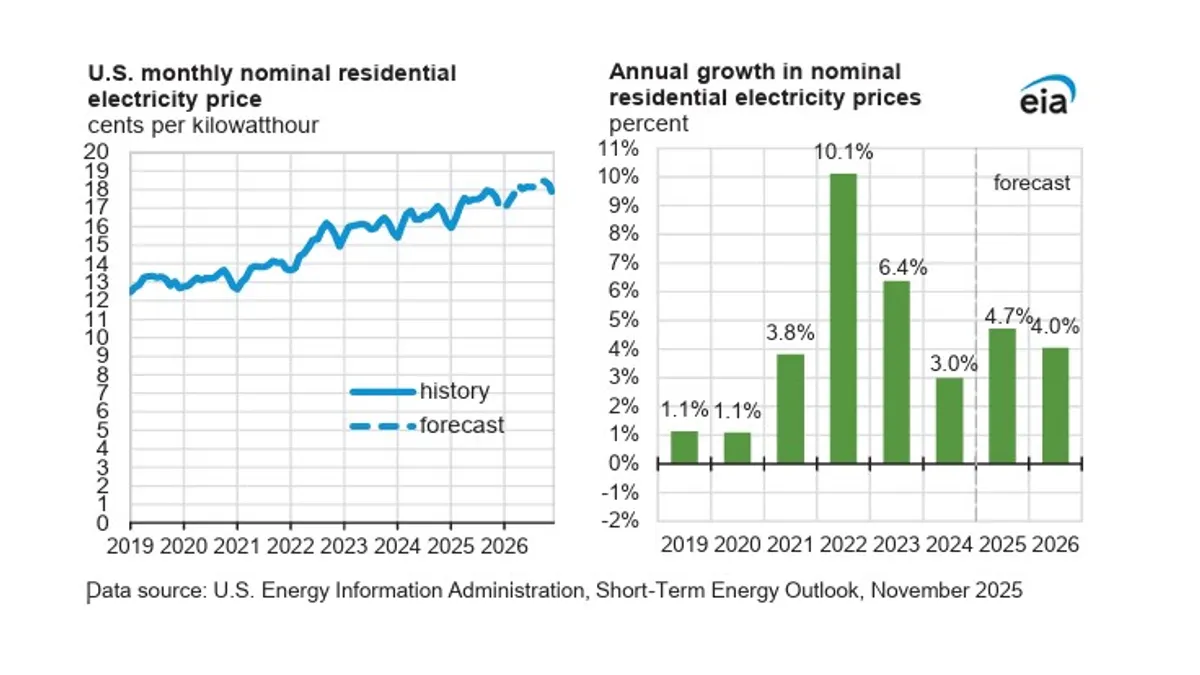
The solar industry is currently going through a time of great change. Consider that during the oil crises in the 1970s, generating solar energy cost about $100 per watt. As research and the use of solar power increased, the price dropped rapidly over the years and then stagnated during the 1990’s as interest faded.
Current concerns about climate change, combined with market factors, have resulted in the price of solar energy taking a nosedive to 50 cents per watt over the last five years. Price reductions have accelerated installations and about 65,000 megawatts of electricity per year are now being installed, generating the equivalent of 65 coal-fired power plants. In the last two years, a tipping point has been reached in solar power as more people are accepting it as a viable energy resource – including politicians and the utility industry.
However, solar energy still needs to be more efficient to compete with natural gas-fired power plants. High installation costs continue to make the transition to solar power prohibitive. To make solar energy more affordable, the panels themselves need to be more efficient so that fewer panels are needed. This is especially important for locations that have limited usable roof space.
Rating Energy Efficiency
There are many types of panels currently being built and researched. Approximately 90% of the solar panels in use are made from crystalline silicon. The National Renewable Energy Laboratory (NREL), tracks the efficiency ratings of all kinds of panels. According to the NREL, the world record for silicon cell efficiency is 25%, but it is measured on a very small cell made of the best—and most costly—materials available. The most commonly used panels made of silicon clock in at 15-21%. Silicon’s ratings haven’t really improved since the late 1990’s.
The most efficient material for solar cells is Gallium Arsenide with ratings charting around 28% but it costs forty times more than silicon. There have been impressive efficiency advances in other materials in the past few years including the two “thin films,” cadmium telluride and copper indium gallium selenide, both of which are up to 22%.
The big news recently is about cells made with perovskite, which came out of nowhere in 2012 and is already showing ratings of 22%. Never in history has a type of semiconductor advanced in efficiency so quickly. Perovskite enthusiasm is based partially on some of the basic tenets of how photovoltaic power is generated.
The semiconductors inside solar panels produce electricity by using sunlight to excite and move electrons from what’s called the “valence band” across a “band gap” to the “conduction band.” To increase efficiency, the band gap has to be the right size. If the gap is too big, the electrons can’t make it across. If the band gap is too short, you get low voltage, which isn’t good either. Higher band gaps give you higher voltages but a lower current level, so it’s a delicate balance to find the right size gap.
The ideal gap is about 1.4 electron volts, the unit of measurement used for band gaps. You can then increase the efficiency in cells by using applications that include “tandems,” where cells with different gaps are stacked on top of each other. By stacking cells you can get the efficiency up to 42%.
Perovskite is a type of salt with a crystalline shape. Methylammonium lead oxide is the most common perovskite that scientists work with, but there are different kinds that produce a variety of band gaps. Using different perovskites allow band gaps to be “custom tuned” to get them the right size. Perovskites absorb well into the other substances, and have a long carrier lifetime and low surface recombination – two physics terms that translate into higher efficiency ratings. Perovskites can also be printed onto plastic—a flexible and inexpensive material. The upshot is the material has a lot of potential for efficiency growth.
Recent Breakthroughs
While more efficient than crystalline silicone, the material is not perfect. More research is needed to resolve current issues that prevent perovskite from becoming an optimal semiconductor source.
Stanford University Professor Michael McGehee and his team have found ways to mitigate these issues. Properly packaging the cells into panels is vital to making the cells work properly. McGehee’s group has tested several of its designs in very rigorous, 1000-hours industry testing methods and all but one passed, so they are very optimistic about the future.
The world record for efficiency is 46%, which was achieved by Soitec and CEA-Leti, France, together with the Fraunhofer Institute for Solar Energy Systems ISE, Germany.i It was achieved using a tandem cell and uses four different band gaps. Keep in mind these are very tiny cells, cost over $40,000 to make and due to their small size would not have much application outside a laboratory setting. The belief is that similar results can be achieved using perovskite for a much lower cost, making them more practical in real world settings
McGehee’s lab recently logged a world record for a two-terminal, tandem design that achieved 23.6% rating, as measured by the NREL. They are also experimenting with different metals, including tin, to help fine-tune their band gaps. There is concern in the scientific community regarding the use of lead in several of their designs. But they are actually using far less lead in their panels than the quantity found in panels currently produced.
The Stanford research team is not alone in pursuing the opportunities offered by perovskite. There are at least 5 other companies currently working with this very promising material. These organizations have come to the same conclusion in their research: perovskite has the potential to become a formidable contender for solar cell efficiency ratings and revolutionize the solar power industry.
If you want to learn more about what’s happening in today’s solar research, Stanford currently offers online professional courses on energy innovation and emerging technologies. Click here for more information.